Almost 45% of the population evaluated, highlighting a significant potential need for pharmacological intervention within this group.
Jorge Velasco Zamora MD CPI MBA
Obesity is a major public health concern associated with elevated risks for over 20 chronic diseases, including type 2 diabetes, hypertension, hypercholesterolemia, stroke, cardiovascular diseases, and various cancers. Additionally, individuals with obesity face heightened risks of joint pain, urinary incontinence, impaired mobility, and notable psychosocial challenges. Analysis from the "Global Burden of Disease" (GBD) study indicates that a body mass index (BMI) of ≥25 kg/m² correlates with approximately 2.4 million deaths in women and 2.3 million in men (2).
The prevalence of obesity has risen markedly over recent decades. Between 1990 and 2022, the global rate of obesity among children and adolescents aged 5 to 19 years increased from 2% to 8%. Similarly, obesity rates among adults (18 years and older) doubled, escalating from 7% to 16% (1) .
Current treatment guidelines emphasize a multidisciplinary approach to obesity management, incorporating lifestyle modification, behavioral therapy, bariatric surgery, and pharmacotherapy. Anti-obesity medications (AOMs) are generally recommended for patients with a BMI ≥30 kg/m², or ≥27 kg/m² when one or more comorbidities are present (3).
While a reduction of at least 5% in total body weight qualifies patients as responders to AOMs, evidence suggests that greater weight loss yields additional health benefits. Weight reductions of 5-10% are linked to decreased risks of various metabolic, skeletal, and anatomical complications. However, for significant cardiovascular (CV) benefits, reductions exceeding 15% may be necessary (4).
Currently, monotherapy for obesity has shown moderate efficacy, except for GLP-1 receptor agonists (GLP-1 RAs), such as semaglutide. When paired with lifestyle interventions, GLP-1 RAs can achieve weight reductions exceeding 15% (5). For more substantial weight loss, combining therapies targeting multiple mechanisms may be required. Tirzepatide, a dual GIP/GLP-1 agonist, has demonstrated potential for inducing weight reductions of approximately 23% at its highest dose (6).
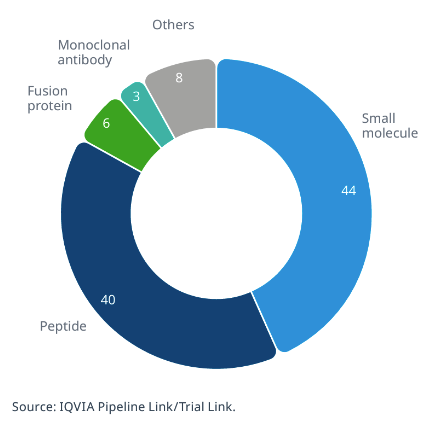
In the coming years, new AOMs are expected to emerge, potentially transforming the therapeutic landscape of obesity and reducing associated morbidity and mortality. As of August 2024, IQVIA Pipeline Link reports 107 ongoing clinical research programs (Fig 1) exploring diverse mechanisms of action in various development stages, from discovery to preclinical and clinical phases.
As of August 2024, IQVIA Pipeline Link has identified 107 clinical research programs (Figure 1) that include diverse mechanisms of action and are in different stages of development, ranging from the discovery stage to the preclinical and clinical phases.
Rationale and Study Design
In response to the growing public health needs and the expanding pipeline of anti-obesity drugs in development, an increased demand for clinical study participants is anticipated. As a research center, we aimed to assess the weight profile of our local population to better understand potential recruitment pools for future trials.
To this end, we conducted a prospective observational study involving 1,399 individuals who attended our institute consecutively. Data collection focused solely on body mass index (BMI), categorized according to the World Health Organization (WHO) classification system (7). Based on these data, we calculated the prevalence and distribution of obesity within the cohort, yielding the results shown in Table 1 and in the next figure.
|
WHO classification |
BMI |
(n) |
(%) |
|---|---|---|---|
|
Underweight |
< 18.4 |
9 |
0.6 |
|
Normal weight |
18.5-24.9 |
275 |
19.7 |
|
Overweigth |
25 – 29.9 |
489 |
35 |
|
Obesity type 1 |
30 – 34.9 |
366 |
26.2 |
|
Obesity type 2 |
35 – 39.9 |
168 |
12 |
|
Obesity type 3 |
≥40 |
92 |
6.6 |
Table 1
Results and Implications
The study revealed that approximately one in five participants (19.7%) had a BMI within the adequate weight range. The most prevalent category among the population was overweight, defined by a BMI between 25 and 29.9 kg/m², representing 35% of the sample and excluding obesity types 1, 2, and 3.
As noted, a BMI ≥ 25 kg/m² correlates with increased mortality rates2 compared to lower BMI ranges. In our cohort, nearly 80% (79.8%) of individuals fell within this category, highlighting a substantial proportion of the population at elevated health risk.
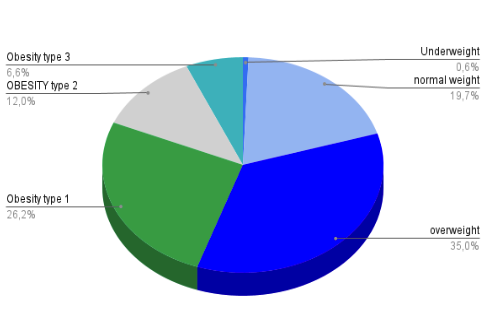
Furthermore, international guidelines3 recommend anti-obesity medications (AOM) for individuals with a BMI ≥ 30 kg/m², which would apply to 44.8% of the evaluated population, underscoring a significant potential need for pharmacological intervention within this group.
This study, however, is limited by its observational, cross-sectional, and retrospective nature, as it only examined BMI and did not account for other risk factors, comorbidities, or concurrent medications.
Dr Jorge Velasco Zamora
Site Manager, Instituto CER
Acknowledgments
We extend our gratitude to technicians Mariana Ocaranza, Natalia Sosa, Christian Amarilla, and María Laura Canizzaro for their invaluable contributions to this study.
About Instituto CER
Located in Quilmes, Buenos Aires Province, Instituto CER is a private clinical research center with over 25 years of experience across diverse medical specialties. The institute’s metabolic disease research team includes experts in gastroenterology, cardiology, immunology and internal medicine.
Instituto CER also founded and sponsors Fundación Articular, a non-profit organization dedicated to raising public awareness in science and technology and promoting early diagnosis of various diseases.
Instituto CER is currently collaborating with the Argentine start-up RadBio Inc. to support research on a novel drug for the treatment of systemic sclerosis.
https://www.institutocer.com.ar/en/
Bibliography
- https://www.who.int/health-topics/obesity#tab=tab_1
- Dai, H. ∙ Alsalhe, T.A. ∙ Chalghaf, N. ∙ et al.The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study PLoS Med. 2020; 17, e1003198
- Apovian, C.M. ∙ Aronne, L.J. ∙ Bessesen, D.H. ∙ et al.Pharmacological management of obesity: an Endocrine Society Clinical Practice GuidelineJ Clin Endocrinol Metab. 2015; 100:342-362
- Group, L.A.R. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial Lancet Diabetes Endocrinol. 2016; 4:913-921
- Wilding, J.P.H. ∙ Batterham, R.L. ∙ Calanna, S. ∙ et al. Once-weekly semaglutide in adults with overweight or obesity N Engl J Med. 2021; 384:989-1002
- Jastreboff, A.M. ∙ Aronne, L.J. ∙ Ahmad, N.N. ∙ et al. Tirzepatide once weekly for the treatment of obesity N Engl J Med. 2022; 387:205-216
- who.int/health-topics/obesity#tab=tab_1


